Data-Driven Health: A Clinical Platform to Prevent Venous Thromboembolism
A comprehensive digital registry for VTE cases with real-time data collection and research capabilities.
The Client
A national medical association focused on hemostasis and thrombosis gathers hematologists, phlebologists, and lymphologists to advance research, diagnosis, and prevention of clot-related disorders. One of its strategic goals was to create a standardized data-collection system that would generate local evidence and inform more effective health policies.
The Challenge
Venous thromboembolism (VTE) is a leading cause of in-hospital complications, yet clinicians had no unified digital registry that reflected day-to-day reality. Without trustworthy local data, it was impossible to benchmark protocols, analyze outcomes, or design targeted prevention programs. The association needed an ethical, easy-to-use tool that blended naturally into routine practice and produced clean, research-grade information.
The Solution
Tech stack used:
We built a web-and-mobile clinical registry tailored to physicians managing VTE cases. From UX to cloud infrastructure, every layer was crafted for speed, security, and scientific rigor.
- Secure, role-based access—only credentialed physicians can add or view cases.
- Built-in digital consent that anonymizes patient data and streamlines ethics compliance.
- Comprehensive clinical record—history, diagnostics, treatment, evolution, and complications.
- Longitudinal updates so physicians can reopen a case and add follow-up data, creating an evolving timeline.
- Private research dashboard ready for interactive analytics and future predictive models.
- Curated article library editable by the association, plus sponsor sections for anticoagulation partners.
See it in action
Built for the future of care
Testimonials
"Capturing real-time data at the bedside changes how we understand this disease. We're finally producing our own evidence."
Key Differentiators
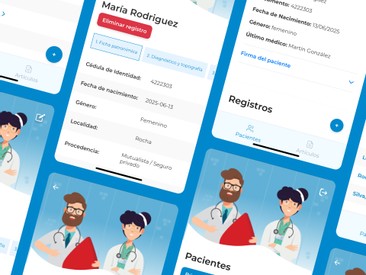
The main beneficiaries include hospitals and clinics aiming to digitalize VTE registries, improving data management and patient care.
Scientific societies also benefit by gaining reliable, real-world data to support the creation of evidence-based clinical guidelines.
Lastly, pharmaceutical companies and insurers can leverage outcome-driven real-world evidence to evaluate clinical results and guide data-informed strategic decisions.
Next Steps
Ready to see it in action?
Or do you have a specific challenge in your medical practice or institution?
Let’s talk